Below is a really interesting technological packaging advancement that is truly exciting and could have major benefits in the healthcare and pharma packaging & clinical trials sectors. Design Cognition also believe that this technology has further application in other dosage forms and sectors and is working with DSM TCG & GP Solutions (UK) Ltd to develop the OtCMâ„¢ and Dose Guardâ„¢ technologies in innovative ways to meet the demands of end-users and industry and bring this product to market in a viable & cost-effective manner.
Please contact me (chris@designcognition.com) if you’d like more information or come and visit us at Packaging Innovations at the NEC, Birmingham UK on 24th or 25th Feb (stand 582), where we can discuss in more detail. You can register HERE. Chris Penfold
When was the last time you forgot to take your medication? Odds are that it was within the last week. This makes you part of the millions of patients who don’t take their medications as prescribed by their physician. Medication non-adherence, or mal-compliance, as it is commonly called, is a problem that disrupts the healthcare system in many ways. If you don’t take your medication odds are that you won’t get the full benefit of the treatment. As former US surgeon general Dr. C. Everett Koop said, “Drugs don’t work in people who don’t take themâ€. In the worst case, you could be among the patient population who are hospitalized as a result medication non-adherence. The cost to the healthcare system? Phenomenal! Mortality count? Sinister! Morbidity rates? Unacceptable!
At its root, this problem, like many, is a problem of human behaviour. Even Hippocrates (460-377 BC), the “Godfather of Medicineâ€, gave early warnings of the non-compliance issues to his students and colleagues. (”Keep watch for that fault in patients which makes them lie about the taking of things prescribed.”). Even though we are “creatures of habitâ€, we often lose momentum when taking medications, especially for chronic conditions.
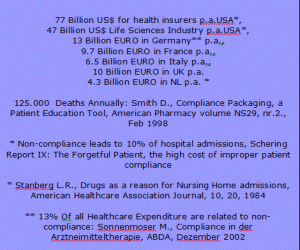
The Non-Compliance Money Waste List
There have been several attempts at using technology to influence compliance rates. As one scans the Web looking for potential help, some solutions that arise include:  (1) A variety of medication reminders that will beep, blip or blurt when it is time to take your medication. (2) A variety of devices that can track the number of times a medication dosage has been removed from a medication pack or a medication container (USA) that has been opened. The data collected can be downloaded at the doctor’s office or pharmacy to check compliance statistics. (3) A variety of devices that can remind the patient to take their medications and confirm that they have taken it by pressing a button to send the data. (4) A miscellaneous group of reminder devices with alarms, like automated pill boxes or wrist watches.
OtCMâ„¢ -Â Objective therapy Compliance Measurement.
One of the weaknesses of these approaches is that one can never guarantee that the patients have taken their medicine after an alarm. In fact, one can’t detect if the medication unit dosage has been taken, or if the medication unit dosage has even been taken at the right time.
A new technology has arrived that has the potential to dramatically impact the therapy compliance rates for individuals, particularly seniors, as they go about their daily lives. The name of this technology is OtCM™, Objective therapy Compliance Measurement, using the most recent RFID/NFC (Radio Frequency Identification, Near Field Communication) technology, including embedded sensor functionalities, and combined with printed organic electronics. The OtCM™ application was invented by Jos Geboers and Willem Kort, who are working in the healthcare industry, especially in clinical pharmaceutical R&D, Health Economics, Outcomes Research and Patient Recorded Outcomes. To that purpose Messrs. Geboers and Kort initiated the foundation of a consortium that includes all players, i.e. top listed pharmaceutical companies, medication packagers, health insurers and Royal DSM N.V.: “The Compliers Group†(DSM TCG). DSM TCG wanted a way to be as certain as possible that patients were taking their medications at the correct frequency each day. They knew that, whatever solution they decided on, it needed to be “real time†since any delays in therapy could have serious health consequences. A novel system for measuring the time and quantity of drugs taken out of conventional, existing medication packaging or medication container, is used. “Leading†versus “Bleeding†edge …
The existing medication package will do …
The system consists of conventional, existing medication blister pack (or “bottleâ€) of a given prescription drug furnished with “organic electronics†(circuitry, power supply), i.e. functional polymers and coatings, to print “the micromechatronic blister/bottleâ€. A “traditional†silicon RFID/NFC IC (Integrated Circuit) that has been especially designed for OtCMâ„¢ with integrated interfaces for i.e. clock and temperature functionality, provide the intelligence to identify data (date-time) of pills/capsules that have been taken out of the blister pack/bottle, whilst wireless Radio Frequency (RF) techniques (NFC, Near Field Communication) are able to transfer data wirelessly from the “OtCMâ„¢ enabled blister/bottle†onto DSM TCG’s webserver.
Driven by the intelligence of the RFID chip, embedded in the “mechatronic circuitryâ€, and the printed power supply (capacitator, battery), an electric current is circulating through the circuitry of the packaging at regular time intervals. As soon as a dosage is removed out of the packaging, the chip is activated. This information is stored. When in close proximity of a (NFC) reader, the recorded information in the packaging will be transferred and stored in the server to populate the Therapy Compliance Database of active patients, under all regulatory recommended & required security and privacy conditions, locally and globally.
The standard existing medication blister package will do at a fraction of the traditional costs!
The concept of using the technology of printing functional polymers and coatings is an innovative approach. A large variety of applications in real-life situations has been brought about, especially for purposes of identification of products and, consequently, the packaging of products. The standard medication blister package will do at a fraction of the traditional costs!  Moreover, until now, traditionally available therapy compliance devices are extremely costly, even if mass-produced. Mass produced RFID-tags combined with polymer printing will introduce low-pricing schedules, based on factors of 90-95% reduction vs. currently available “traditional electronic†OCM devices, i.e. pill boxes or so-called “smart pill boxesâ€.
DSM TCG’s OtCMâ„¢ technology can be combined with GP Solutions (UK) Ltd’s patented and approved Dose Guardâ„¢ solution; a child resistant senior friendly secondary barrier that when applied to a blister pack will render it safe for use. Both “come with the standard, existing medication packageâ€. From surveys it has been identified that patients and health practitioners (physicians, medical specialists, pharmacists) only appreciate the enabling of OtCMâ„¢ right from the medication packaging. There obviously is no need for an additional, “stand-alone†therapy compliance measurement (recording) device separated from the medication package.
During OtCMâ„¢ we measure through the standard medication packaging:
(1) when the pill/capsule is taken,
(2) the location of the removed pill/capsule onto the blister packaging,
(3) the correct dosing schedule,
(4) an acoustic signal might prompt the for action, data re: expiration date,
(5) production information re: temperature, batch IDs from the production line, drug interaction alerts.
This real-time “interventional approach†is what stands apart from other compliance solutions. In conclusion, it is clear that there are several technological approaches that are aimed squarely at the problem of medication compliance. Though these advances give our “inner geek†some encouragement, they are ultimately targeted at making sure that at-risk populations stay on track as they take medications to improve their health status. David Rosa, Willem Kort
You can find further information on compliance and evolving technologies, via Design Cognition’s sister site ‘The Pharma Gateway‘. Also, if you are interested in our forthcoming compliance workshops – let us know.
Contact Chris Penfold (chris@designcognition.com).

