The normal packaging development process would involve firstly developing a pack to fit the product in an optimum manner. Then any legally required text would be positioned (including any required symbols, such as recycling logos). Finally the marketing requirements would be implemented, in terms of claims, branding etc. More often than not, there is then a need to go back to product marketers to get them to reduce their marketing copy on the packs, because it simply won’t fit……..and the situation is getting worse!
Other options that can help tackle this problem are:
A, reducing the font size – but this can have implications on readability, print process constraints and there is often a legal minimum size which needs to be adhered to.
B, Using paginated label leaflet formats – where, on occasions, we have developed up to a 10-page concertina style leaflets. However, these multi-layered add-ons can add a huge on-cost onto the price of a pack – for which marketers don’t always want to pay.
C, Printing on the inside of pack (e.g. cartons) – but not visible until a pack is opened. So one can’t do this with certain text which needs to be visible at time of purchase
D, Finally, if all else fails, considering increasing the overall size of the packaging.
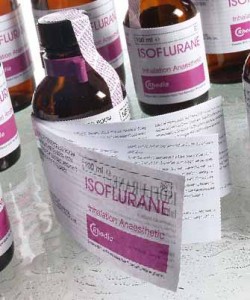
An example of a multi-page label solution
This latter route is sometimes unavoidable, especially with pharmaceutical packs, where the packs are very small, and even after taking measures such as reducing the bar codes from 13 digit to 8 digit, there is simply not enough room to display the mandatory legal minimum required text (let alone any marketing text).
Furthermore, it is now the law for any new medicines to incorporate Braille on the packaging (it will be a requirement for all existing medicines by October 2010). This will have to detail the product name, the strength [of the medication] and the dose form – yet another constraint to bear in mind. New products also have to conform with the readability guidelines which are in place to ensure that the packs can be read clearly and understood by the patient/consumer).
It’s worth remembering that it’s not just the basic information that one has to put on the pack. Very this has to be repeated it in a number of different languages. It’s not unusual for a European product to have a need for 12 languages. And then there might be a need to repeat ‘country of origin’ for every language, and could require five countries needing to be listed (for a range of ingredients). The result could be a situation where all one has room to display is a list of ingredients & addresses, and any wish for aesthetic beauty just ‘goes out of the window’.
At Design Cognition, we review the whole space to find an ideal design that looks most aesthetically appealing and hopefully doesn’t look too cluttered – but it’s not always easy!
Incorporating logos of different colours, or trying to mix varying colours of text with backgrounds, can add its own issues and problems. Sometimes ‘house colours’ do not lend themselves to readability, White text on a pastel or black background for instance, can be even harder to read, so we may need to redesign [the pack graphics], using the principles and processes detailed above.
Overall our job is to develop a pack that is fit for purpose and not over-packaged. Things are becoming increasingly challenging but, so far our use of creativity and lateral thinking has provided a suitable solution.
Moving forwards technology could aid some of the issues raised above. Nowadays, certain consumer/marketing information can be shown ‘on-line’, cutting down the need to put it all on-pack – maybe just a web address for further information. There have also been great leaps forward in microchip technology which will enable ‘talking packs’, ‘moving pictures’ and a whole new interactive consumer experience – taking packaging to a new level. These are all areas in which Design Cognition has a strong interest and is working with a number of suppliers to develop cost-effective solutions.
If you’d like more information on these areas, sign up for further information HERE
Chris Penfold